Technical
OEM Information
Battery Basics
Questions
and Answers – What you need to know.
What exactly is a
battery?
A battery is a source
of electrical energy. Its smallest unit is called a (galvanic) cell. A
battery usually consists of several individual cells electrically connected
in series. The chemical energy as stored in each cell is converted directly
into electrical energy when its terminals are connected to an electrical
consumer.
A galvanic cell also
needs two substances for energy conversion, represented by two electrochemically
active electrodes of different composition, both of which are immersed
in an electrolyte which provides a conductive medium between them.
One of the electrodes
uses a metal such as zinc or lithium. Within the electrolyte it establishes
a negative potential and consequently represents the negative electrode.
The other electrode consists of an electron conducting compound which
is rich in oxygen, e.g. manganese dioxide, silver oxide, nickel hydroxide
or atmospheric oxygen in combination with a suitable oxygen electrode.
This electrode establishes a positive potential within the electrolyte
and consequently represents the positive electrode of the electrochemical
system. Depending on the electrochemical system, the cell voltage will
be between 1.2 V and 4 V. When connecting the system to an external load,
electrical energy will be taken out of the system, while the chemical
energy stored inside the cell or battery will be used up.
What
is the difference between a cell and a battery?
The smallest electrochemical
unit of a battery is called a cell. The cell does not yet have a completed
housing or ready-to-use contacts, and is usually connected with its neighboring
cell within the battery via soldered or welded contacts.
In contrast to a cell,
a battery is easily recognized by its completed housing fitted with ready-to-use
contacts. Furthermore the housing is clearly labeled with the manufacturer's
name, type designation, battery voltage, etc. Since single cells are frequently
offered with the above attributes of a battery, it has also become customary
to refer to this type of single cell as a battery
What
is the difference between a primary and rechargeable battery?
The kind of electrochemical
system decides whether the system is a rechargeable one or not. Truly
rechargeable systems are reversible systems with regard to their electrochemistry
and the structure of their electrodes. In an ideal system, this reversibility
must not be affected by the number of charges and discharges (cycles).
Since charges and discharges also cause a reversible change in electrode
volume and structure, the design of a rechargeable battery must be adequate
to accommodate these changes. Since a primary battery undergoes just one
discharge, its internal construction is generally more simple as it does
not have to accommodate reversible volume changes. No attempt should be
therefore made to recharge primary batteries. This is both dangerous and
uneconomical.
If a rechargeable
system is required, the only sensible choice is to select a truly rechargeable
system which permits more than 1,000 safe cycles. Batteries of this type
are also referred to as Secondary Batteries or Accumulators.
Other marked differences
between rechargeable (secondary) and primary battery systems are their
weight-related energy content, the weight-related load capability and
the rate of self-discharge.
The weight-related
and volume-related energy density of primary batteries is generally far
higher than secondary batteries, whereas their load capability is relatively
small. Secondary batteries have a higher load capability. Rechargeable
lithium-ion batteries, as developed in recent years, boast a high weight-related
energy content. The rate of self-discharge is favorably low for all primary
batteries, regardless of system.
Why do batteries have
different voltages and capacities?
Different devices
operate at different voltages and power levels. They all require batteries
that provide the necessary power output at a minimum discharging voltage.
The voltage of a given battery depends on the number of single cells connected
in series and on their electrochemical system. For instance, a lithium-manganese-dioxide
cell has a nominal voltage of 3 V, a rechargeable lead-acid cell offers
2 V, while an alkaline-manganese cell has an initial voltage of approx.
1.5 V, that decreases during discharge to 0.9 V and below.
The capacity of a
battery is determined by the amount of chemical energy stored inside its
housing. It determines - for a given current of a given device - the service
life of the battery.
In order to properly
operate a specific electrical device,
- the battery's operating
voltage must be matched to that of the device;
- the correct battery
capacity must be selected in order to provide the necessary operating
time for the device;
- the battery must
be able to deliver the power required: its internal resistance must
be smaller than that of the device.
Which
voltages are typical for which types of batteries?
|
Battery Type
|
Voltage
|
Most Common
Applications
|
|
SLI-battery
(starter battery)
(secondary battery)
|
12V,
6V
|
Automobiles,
commercial vehicles, motorcycles
|
|
Semi-traction
battery
(secondary battery)
|
12V,
6V
|
Electric vehicles,
wheel-chairs, lawnmowers, boats, house trailers, cleaning equipment,
solar technology
|
|
Lithium bloc
(2 series-connected
cells)
|
6V
|
Cameras
|
|
Lithium-manganese
button cell
|
3V
|
Pocket calculators,
watches, remote control devices
|
|
Silver-oxide
button cell
|
1.55V
|
Wrist watches,
small clocks
|
|
Alkaline-manganese
round cell
|
1.5V
|
Audio equipment,
cameras, games
|
|
Alkaline-manganese
button cell
|
1.5V
|
Pocket calculators,
electronic devices
|
|
Zinc-carbon
round cell
|
1.5V
|
Alarms, flashlights,
toys
|
|
Zinc-air button
cell
|
1.4V
|
Hearing aids
|
|
Mercury-oxide
button cell
|
1.35V
|
Cameras, hearing
aids
|
|
Nickel-cadmium
cell
(secondary battery)
|
1.2V
|
Power tools,
camcorders, mobile telephones, cordless telephones
|
|
Nickel-metal-hydride
cell
(secondary battery)
|
1.2V
|
Mobile telephones,
cordless telephones, camcorders, notebooks
|
|
Lithium-ion
cell
(secondary battery)
only available
as power pack
|
4V/cell
|
Mobile telephones,
notebooks, camcorders
|
How long may batteries
be stored idle?
In principle, no battery
can be stored without loss of energy, although some battery systems may
be stored for longer periods of time than others. Processes inherent to
the battery's electrochemical system cause a gradual, but unavoidable
loss of usable energy which, however, is predictable. The best known process
is "self-discharge". This generally has to do with the electrolytic solubility
of the positive electrode material or its thermodynamic instability (e.g.
spontaneous decomposition).
Self-discharge in
rechargeable batteries (secondary batteries, accumulators) is particularly
high in comparison to primary batteries. At room temperature the rate
of self-discharge is in the range of 15% to 25% per month, depending on
the system. Of the rechargeable systems, solar batteries have an unusually
low self-discharge rate of only 10% per month.
Electrochemical self-discharge
in primary batteries is considerably lower, and may even be below 2% per
year at room temperature. However, various processes take place in parallel
with this which lead to an increase of the battery's internal resistance
during storage. These processes lead to a reduction in load capability.
Loss of usable energy becomes noticeable only at relatively high discharge
rates (e.g. motor applications, flashlights etc.). This effect, however,
has nothing to do with self-discharge. At low discharge rates the increased
internal resistance which occurred during storage will not be detectable.
Under normal storage
conditions, the following approximate values apply to self-discharge:
|
System
|
Self-discharge
|
|
Alkaline-manganese
(MnO2/Zn), round cells
|
max. 2%/year
|
|
Zinc-carbon
(MnO2/Zn, slightly acid), round cells
|
max. 4%/year
|
|
Lithium (Li/MnO2),
round and button cells
|
approx. 1%/year
|
|
Accumulators
(dependent on system)
|
10% to 25%/month
|
What
is the best way to store batteries?
A general rule is:
The higher the storage temperature, the worse the capacity retention and
vice versa. A refrigerator, with a temperature range from 0°C to 10°C,
is a good place for storing batteries, especially primary batteries. The
refrigerator may, of course, also be used to store secondary batteries,
but since they are rechargeable, their loss of capacity during storage
may be better compensated by recharging, particularly as they can take
up substantial space in the refrigerator (e.g. automotive batteries)
How
can we measure a battery's energy output?
The electrical energy
E delivered by a battery to an electrical device may be computed by formula
E = V x I x t, where
V is the battery's discharging voltage (in volts), I is the discharging
current (in amperes) and t is the time of discharge (in hours). The unit
of energy E as computed by the above formula is given in watt x hours.
"Power
output " of a battery: What does it mean?
A battery's power
output refers to its ability to deliver a specific amount of energy within
a fixed period of time. The power output P of a battery is calculated
from the product of the discharging current I (measured in amperes) and
the discharging voltage V (in volts), thus : P=I x V. The power output
is expressed in watts.
The smaller a battery's
inner resistance, the higher its possible power output. Its inner resistance
must always be smaller than that of the electric device to be operated.
Otherwise the battery voltage would break down, i.e. the battery would
be unable to operate the device. At a given discharging voltage, a battery's
power output increases with increasing electrode surface and operating
temperature, and vice versa.
|
Formulas and
Relationships:
|
|
Cell Capacity
|
IT
|
|
Cell Energy
|
Wh = PT =
IVT
|
|
Power
|
P = IV = I2R
|
|
Resistance
of a Conductor
|
R = V/I (0hms
Law)
|
|
Current (Cells
in Parallel)
|
I = I1
+ I2 + ...In
|
|
Total Resistance
(Cells in Parallel)
|
1/R = 1/R1
+ 1/R2 +...1/Rn
|
|
Voltage (Cells
in Parallel)
|
V = V1
= V2 =...Vn
|
|
Current (Cells
in Series)
|
I = I1
= I2 = ...In
|
|
Total Resistance
(Cells in Series)
|
R = R1
+ R2 +...Rn
|
|
Voltage (Cells
in Series)
|
V = V1
+ V2 +...Vn
|
|
I =
|
Current expressed
in amperes
|
|
V =
|
Electromotive
force (emf) expressed in volts
|
|
P =
|
Power expressed
in watts
|
|
R =
|
Resistance
expressed in ohms
|
|
Wh =
|
Watt-hours
|
|
T =
|
Time expressed
in hours
|
What
are "dry" and what are "liquid" batteries?
The terms "dry battery"
and "liquid battery" are restricted to primary systems and date from the
early development of galvanic elements. At that time, a liquid cell consisted
of an electrolyte-filled glass container into which electrochemically
active electrodes were immersed. It was only later that unspillable cells
which could be used in any position and had a completely different construction
were introduced, these being similar to today's primary batteries. These
earlier cells were based on paste electrolytes. At that time they were
known as dry batteries. In this sense today's primary batteries are also
dry batteries.
The term "liquid battery"
is in principle still applicable to certain modern secondary batteries.
For large stationary lead-acid or solar batteries, liquid sulfuric acid
is preferred for the electrolyte. For mobile applications unspillable,
maintenance-free lead-acid batteries are recommended and have been available
for many years. Their sulfuric acid is immobilized by a gel (or a special
microglass mat).
What
is the influence of temperature on battery performance in general and
of humidity in zinc-air batteries in particular?
Of all environmental
factors, the temperature has the greatest effect
on battery charge and discharge behavior. This has to do with the
temperature- dependent electrochemical reactions occurring at the electrode/electrolyte
interface, which may be considered the heart of the battery. If the temperature
decreases, the rate of electrode reaction decreases too. Assuming the
battery voltage remains constant, the discharging current drops and thus
the power output of the battery. The opposite effect occurs if the temperature
rises, i.e. the power output of the battery increases.
The temperature also
affects the speed of transport processes within the electrolyte and its
porous electrode. A rise in temperature accelerates transport processes,
a decrease in temperature slows them down. The charge / discharge performance
of the battery may also be affected.
The effect of the
relative humidity depends on the battery system. It plays a key role in
"open" battery systems (unlike closed battery systems). Its effect can
also be crucial, as in zinc-air batteries (most frequently used for powering
hearing aids). The unique feature of a zinc-air cell is that it is in
direct contact with the surrounding atmosphere. It will therefore begin
to dry out if the atmosphere is too dry. If the relative humidity of the
environment is too high, the system will begin to pick up water. Both
scenarios have an adverse effect on the performance of the zinc-air cell.
What
consequences can a battery short circuit have?
An "external short
circuit" can occur if the external terminals of a battery are bridged
by any kind of conducting material. Depending on the battery system, a
short circuit may have serious consequences. For example, the temperature
of the electrolyte may rise, thus building up an internal gas pressure
which may open the pressure valve of the battery and eject electrolyte
from the battery. This can cause injuries. In extreme cases a detonation
may even occur if the safety vent fails to respond (due to e.g. a molding
defect during production).
You should therefore
ensure that, for example, you do not carry charged or fresh batteries
in the same pocket as coins or bunches of keys. Otherwise they may bridge
the battery's terminals.
It is also important
to avoid mechanical impacts which could deform the battery and result
in internal electrode short circuits with the consequences described above.
What
are "portable batteries"?
A portable battery
is primarily a battery which provides electrical energy to operate portable,
cordless equipment. In a more generalized definition it also includes
batteries that only operate certain sub-devices within a larger system
(which may be operated by the mains), e.g. a desktop computer. Sub-devices
of the above kind may be a computer's internal clock or a memory backup.
Larger batteries (e.g. four kilograms and above) are no longer considered
portable. Today's typical portable batteries will weigh several 100 grams.
The portable battery family includes both primary and rechargeable (secondary)
batteries. Button cells belong to a special group of their own.
Do today's portable
batteries still have a leakage problem?
"Leakage" (visible
loss of electrolyte) was an annoying, discharge-related phenomenon of
zinc-carbon batteries, which were one of the most popular battery types
until the end of the 1970s. The zinc can of this system, which serves
as the anode, was perforated by the electrochemical oxidation of the zinc,
thus causing the electrolyte leakage. Furthermore, zinc chloride was chosen
as the prevailing electrolytic component. This produces a reaction product
that absorbs water. Under extreme conditions, however, a zinc-carbon battery
may still leak, if e.g. a flashlight is left switched on for weeks or
months. Even if it no longer provides any light, the electrochemical reaction
continues as long as positive electrode material is available.
A further improvement
in the area of leak-proof batteries was achieved by the development of
the alkaline-manganese battery (also called ALKALINE). For some years
now, Varta has been selling only alkaline-manganese and zinc-carbon primary
batteries of improved design and chemistry. Today, all portable batteries
are leak-proof under normal operating conditions.
PRIMARY
(NON-RECHARGEABLE) PORTABLE BATTERIES
Do
alkaline-manganese batteries really last longer than zinc-carbon?
Yes, they do. The
alkaline-manganese battery has nearly twice the energy content of a zinc-carbon
battery of the same size, even at higher loads. This battery is particularly
suited for continuous discharge. For low power applications (such as transistor
radios) or applications using discontinuous discharge regimes (e.g. flashlights),
the zinc-carbon battery still represents an interesting and inexpensive
alternative. The on-load period should preferably not exceed five minutes
at higher loads. This limitation does not apply for the more expensive
alkaline-manganese batteries.
How
can the various types of primary batteries be identified?
Batteries standardized
by the IEC (International Electrotechnical Commission) have a clear, internationally
valid designation. However, the use of this designation is voluntary,
so it will not necessarily appear on every primary battery. Nevertheless,
the manufacturer's designation and the battery voltage are always printed
on the battery housing. Reference is often made to the battery's electrochemical
system. The way it is specified, however, may vary from manufacturer to
manufacturer.
Can
primary alkaline-manganese batteries be recharged?
An alkaline-manganese
round cell can be "recharged" about 20 times. In reality, however, this
is not a true recharge process as offered by secondary batteries, because
they do not permit a normal deep discharge like a true rechargeable battery,
but only a partial discharge. Consequently, the recharge process is also
only a partial one, and which therefore should be better called "regeneration"
to differentiate it from a true recharge as offered by secondary batteries.
The serious limitation of its charge/discharge behavior and its very short
"cycle life" renders the regeneration of an alkaline-manganese battery
rather uneconomical.
Various conditions
must be met in order to ensure the successful regeneration of alkaline-manganese
batteries:
1. A "regeneration"
is possible only if a maximum 30% of the battery's initial capacity is
withdrawn at moderate discharge rates, whereby the discharging voltage
should not drop below 0.8 V. When removing more than 30% of the capacity,
an irreversible manganese dioxide structure will develop that prevents
any further "regeneration". The "30% capacity point" and the 0.8 V discharging
voltage can only be monitored by use of proper measuring instruments,
which the average consumer does not possess.
2. Alternatively,
the user would need to buy a recharger for performing regeneration. Other
charging devices like chargers for rechargeable nickel-metal-hydride or
nickel-cadmium accumulators should never be used, because their charging
current may be too high and may lead to gas generation inside the battery,
which in turn may lead to the safety vent opening and electrolyte being
ejected. In extreme cases an explosion may even occur if the safety vent
fails to respond (due to e.g. a molding defect during production). Cases
like this happen very rarely, nevertheless they can happen, especially
if the battery is not used properly.
3. The length of time
needed for "regeneration" (approx. 12 hours) is out of all proportion
to the discharge time (approx. 1 hour).
4. After about 20
partial cycles at the very latest, the battery's capacity will have dropped
to about 50% of its initial value.
5. If a given device
needs more than three batteries connected in series, an additional problem
will arise if the batteries have differing capacities as a result of "regeneration".
This can lead to a voltage reversal of the weakest battery. This danger
is particularly likely if regenerated batteries are used together with
fresh ones. A battery reversal leads to hydrogen evolution inside the
battery, with the danger that unacceptably high pressures will build up.
This can result in electrolyte being ejected and even an explosion!
Regeneration of primary
batteries is not only uneconomical in the long run, but bears in itself
a safety risk. To avoid these risks it is better to use fresh primary
batteries or secondary batteries (accumulators) rather than to regenerate
primary ones.
The latest in RAM
(Rechargeable Alkaline Manganese) battery technology claims to produce
more cycle life than ever before possible, however there are not many
consumer applications where shallow discharge is practical and consumers
will nearly always totally drain the batteries before regeneration. This
fact makes it difficult for RAM batteries to ever be economical.
SECONDARY
(RECHARGEABLE) PORTABLE BATTERIES
What
is characteristically for a rechargeable portable battery?
Every battery constitutes
an electrochemical energy converter capable of converting stored chemical
energy directly into electrical energy. In the case of a secondary battery
(also called "accumulator") - a technology which also includes rechargeable
portable batteries - the chemical energy - as converted into electrical
energy during discharge - may be restored by a charging process during
which electrical energy is reconverted into chemical energy. This procedure
(cycle) can be repeated for more than 1,000 times.
Rechargeable portable
batteries are available in various electrochemical systems, i.e. the lead-acid
system (2 V/cell), the nickel-cadmium system (1.2 V/cell), and the nickel-metal-hydride
system (1.2 V/cell). One new development is the rechargeable lithium-ion
battery (3.6 V/cell), which is not yet generally available, however. This
system has both a relatively high energy density and a high load capability.
Its discharging voltage decreases with the discharge depth. Typical for
rechargeable portable batteries with alkaline or acid electrolyte is their
relatively constant discharging voltage. This breaks down rapidly at the
end of the discharge procedure.
What are the advantages
and disadvantages of rechargeable portable batteries?
Rechargeable batteries
have the advantage of a long service life. They can be recharged more
than 1,000 times. Even though they are somewhat more expensive than primary
batteries, they become quite economical for frequent use.
Rechargeable portable
batteries have a lower capacity than same-sized alkaline-manganese or
zinc-carbon batteries, i.e. they discharge faster. Another disadvantage
of the "rechargeables" is that due to their nearly constant discharging
voltage, it is difficult to predict when end of discharge will come. When
end of discharge is finally reached, its voltage may collapse unexpectedly
- a fact that can have particularly annoying consequences, e.g. when using
cameras. On the other hand, rechargeable batteries offer a higher load
capability than most primary batteries. For Example, the latest NiMH technology
in the most popular AA size battery is typically aimed at the once non-feasible
high drain consumer applications such as digital cameras. This is coupled
with the fact that primary batteries are usually disposable and secondary
batteries may be recharged and used in some cases more than 1,000 times.
(insert discharge curves)
Recently developed
rechargeable lithium-ion power packs may provide additional opportunities
for camera applications. Their characteristics include high load capability,
high energy density and a decrease in discharging voltage with increasing
discharge depth (from 4 V to about 3 V).
Is
it true that rechargeable 1.2 V portable batteries cannot always be used
in units designed for 1.5 V alkaline-manganese batteries?
The answer is no.
The alkaline-manganese battery discharges over the voltage range 1.5 V
(fresh) and 0.9 V (final discharging voltage according to IEC), whereas
rechargeable portable batteries (nickel-cadmium or nickel-metal-hydride)
discharge at a virtually constant voltage of 1.2 V/cell. This voltage
level is roughly equivalent to that of the average discharging voltage
of an alkaline-manganese battery. Therefore, exchanging a rechargeable,
portable battery for an alkaline-manganese battery or vice versa should
never be a problem.
For
which type of application are rechargeable batteries the better choice?
Rechargeable batteries
are particularly well-suited for devices requiring a relatively high power,
i.e. for devices requiring high energy development over a short period
of time, e.g. portable cassette players, portable Compact Disc players,
portable cassette radios, electronic games, motor-driven toys, various
household appliances, professional cameras, camcorders, mobile telephones
or cordless telephones, notebooks, and other equipment having medium to
high power requirements.
Rechargeable portable
batteries are not recommended if a device is rarely used (a rechargeable
battery loses up to 1% of its capacity every day). On the other hand,
a rechargeable battery may be necessary if the device's power consumption
is too high to be met by an alkaline-manganese battery. Rechargeable batteries
have far higher power reserves than alkaline-manganese batteries. In general
it is wise to follow the appliance manufacturer's guidelines for battery
selection as given in the operating instructions.
Which types of rechargeable
batteries are available? For which applications are they especially suitable?
|
Battery Type
|
Characteristics
|
Applications
|
|
Nickel-metal-hydride
round cell
|
- high capacity
- environmentally
benign (contains no mercury, cadmium or lead)
- overcharge-proof
|
Audio devices,
camcorders, notebooks, mobile telephones, cordless telephones
|
|
Nickel-metal-hydride
prismatic cell
|
- high capacity
- environmentally
benign
- overcharge-proof
|
Audio devices,
camcorders, notebooks, mobile telephones, cordless telephones
|
|
Nickel-metal-hydride
button cell
|
- high capacity
- environmentally
benign
- overcharge-proof
|
Mobile telephones,
cordless telephones
|
|
Nickel-cadmium
round cell
|
- robust
- high load
capability
|
Audio devices,
power tools
|
|
Nickel-cadmium
button cell
|
- robust
- high load
capability
|
Memory backups,
cordless telephones
|
|
Lithium-ion
power pack
|
- high load
capability
- high energy
density
|
Mobile telephones,
notebooks, camcorders
|
Is
the power output of a rechargeable portable battery influenced by extreme
temperatures?
Yes, this depends
on the system. At temperatures below -15°C a drop in power output is quite
noticeable for nickel-cadmium and nickel-metal-hydride batteries. At -20°C
the alkaline electrolyte reaches its freezing point. The maximum permissible
temperature for the charging process is +45°C. Above this temperature
the charge acceptance is reduced.
PROPER
CHARGING METHODS OF PORTABLE BATTERIES
What
are the important points to remember when purchasing a charger?
The charger must meet
the requirements of the battery system (see description). A low price
is not necessarily the best argument for buying a charger. Low-quality
or unapproved chargers of unknown makes may damage the battery, thus obliterating
the original savings.
A good charger has
the following features:
- Quick charging
mode: In order to use battery operated devices extensively, it is often
important to be able to recharge batteries in one or two hours.
- Overload protection:
Good chargers have a timer or a temperature sensor. With these features,
the charging process ends as soon as the battery has been fully charged.
Unnecessary overloading can thus be avoided.
Furthermore, good
chargers carry the manufacturer's name and type designation and are sold
together with a relevant technical data sheet and instructions.
What
are the important points to remember when charging a battery?
Some practical tips:
- Never charge primary
batteries! High currents could lead to leakage or even an
- Insert the rechargeable
battery correctly into the charger - note the polarity!.
- Charge secondary
batteries to 100% of their rated capacity prior to first use (they are
uncharged at the time of purchase). It is best not to use the quick-charge
mode when charging them the first time.
- Make sure that
secondary batteries are always fully charged. In order to achieve the
maximum operating time (i.e. maximum capacity), it is essential that
nickel-cadmium batteries are discharged completely before being recharged.
Maximum capacity may not be realized after initial charging or when
charging the batteries after a longer idle period. The battery's maximum
capacity will be restored following repeated cycling (full discharge
and recharge).
- Do not charge rechargeable
batteries at temperatures below 0°C. After charging, however, they may
be discharged at lower temperatures.
- As a general rule
with NiCd and NiMH 1.2V per cell technology you have to input 140% of
the batteries rated capacity to fully charge that battery.(i.e. 140%
in to get 100% out allowing for a condition known as thermal runaway).
This is usually why batteries are charged at the 0.1C rate for 14-16
Hours on standard charging parameters. (10% of cells rated capacity
(0.1C) for 14-16 hours).
- When charging at
rapid rates ( e.g. 1C rate = 100% of rated capacity) it is usually recommended
that 120 – 130% input is only needed.
At -20°C discharge
becomes difficult because the electrolyte freezes at this temperature.
What
does "memory effect" mean?
If nickel-cadmium
batteries are recharged before they have been fully discharged, cadmium
crystals can form at their negative electrodes. This results in an unwanted
second discharge stage. The battery stores this stage as a discharge stage
for the next cycle in its memory, even though capacity is still available
'below' this. During the next discharge process, the battery only remembers
this reduced capacity. Any further incomplete discharge cycles which follow
will aggravate this situation still further and the performance of the
battery will continue to fall. Nickel-cadmium cells should therefore be
discharged fully at occasional intervals. This prevents the 'memory effect'
from occurring and prolongs the service life of the cell or battery. This
effect does not occur with nickel-metal-hydride batteries. Consequently,
these batteries can be discharged and recharged without problem.
The memory effect
is a phenomenon which can quickly end the useful service life of a Ni-Cd
battery if handled incorrectly. The technical explanation for this is
as follows: If you trickle charge a Ni-Cd battery (with low current) or
charge it before it is fully* drained (i.e.perform only partial discharges),
certain chemical compounds are formed on the negative electrode. If you
continue to charge the battery like this, the compounds build up. This
has the effect of gradually reducing the available energy until the battery
only supplies the required voltage for a few minutes.
| |
Discharging
|
Charging
|
Discharging
|
Charging
|
|
Partial discharge:
When re-charging, the available energy is gradually reduced:
Memory Effect
|

|

|

|

|
|
Full discharge:
Battery receives full energy by re-charging:
sufficient voltage for operating the device is granted.
|

|

|

|

|
The memory effect
is linked with the properties of the negative Cadmium electrode, and thus
only occurs with Nickel-Cadmium batteries. You should never recharge these
on an ongoing basis, but should always allow them to be drained until
the device ceases to operate.
|
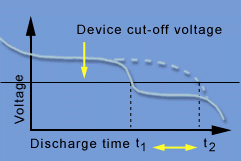
t1-t2: Capacity loss caused by memory effect
|
You have then
reached what is known as the device cut-off voltage. Only then should
you start to charge the batteries. It is easy to discharge a battery
by operating a device until it no longer receives sufficient voltage.
It is even easier to do this by using a charger with a discharge
function.
If a Ni-Cd battery should be rendered inactive by the memory effect,
it can be reactivated by deep-discharging it using a special "refreshing"
device. The memory effect is thus reversible.
|
* The expression "fully"
implies draining the battery until the device cut-off voltage is reached.
With round cells, this is between 0.8 and 1.0 Volt. Draining a battery
completely to the voltage of 0.0 V may destroy the battery.
May
any charger be used to recharge portable batteries?
No, because each charger
employs a specific charging technology which is matched to a given electrochemical
system, e.g. lithium-ion, lead-acid or nickel-metal-hydride. They differ
not only in their voltage characteristics, but also in their charging
mode, e.g. only quick chargers which have been specially developed for
nickel-metal-hydride batteries will ensure optimal charging results for
this system. Former chargers for nickel-metal-hydride batteries can continue
to be used, but may need more time to fully charge the battery. Care must
be taken when using a charger that does not meet the required charging
conditions for a given electrochemical system, even if it carries a label
that seems to signal "officially approved". A label of this kind may only
state that the device was wired according to the county of manufactures
standards and approval ratings. This type of label does not make any reference
to the charger's suitability for a specific battery system, but only complies
with the laws and standards of the country that the charger is sold in.
With cheap devices of this kind, charging nickel-metal-hydride batteries
can be both dangerous and lead to unsatisfactory results. This warning
also applies to chargers developed for other systems (e.g. lead-acid accumulators).
Is
it possible to overcharge secondary batteries?
No - not if a high-quality
charger is employed. High-quality chargers have either a timer or thermo-sensor
to ensure that the charging process is terminated as soon as the battery
is fully charged. This eliminates any risk of overcharge.
Yes - if chargers
of unknown quality and with insufficient instructions may not meet these
requirements. If used, the battery may overheat due to overcharging and
may be damaged as a result.
BUTTON
CELL BATTERIES
What
is a button cell?
A "button cell" should
actually be better called a "button battery", because it has the external
attributes of a battery. Its popular name, however, is "button cell".
A button cell may be defined as a battery whose diameter is equal to or
larger than its height. Present dimensional limits for button cells using
an aqueous electrolyte range from a) diameter: 4.8 mm to 11.4 mm, b) height:
1.05 mm to 5.4 mm. Depending on the electrochemical system their nominal
voltage is either 1.2V, 1.35V, 1.4V, 1.5V or 1.55V. Batteries of this
family were given this name because of their visual similarity to buttons.
Coin Cells also belong to the group of button cells.
What
types of button cells exist? What applications are they suitable for?
|
Button type
|
Characteristics
|
Applications
|
|
Silver-oxide
|
- high, constant
voltage
- self-discharge
below 5% per year
|
Watches, cameras,
pocket calculators
|
|
Alkaline-manganese
|
- provides
a relatively high current
- voltage decreases
with discharge
- self-discharge
below 3% per year
|
Electronic devices,
pocket calculators, low price watches
|
|
Lithium-manganese
|
- very low
self-discharge (< 1% per year)
- low load
applications only
|
Pocket calculators,
watches, remote controls
|
|
Zinc-air
|
- high capacity
- self-discharge
3% per year if not activated
|
Hearing aids,
pocket paging devices
|
|
Zinc-mercury
(Production
to cease in the near future)
|
- provides
relatively high currents
- self-discharge
2% per year
- harmful to
environment if disposed of incorrectly in large quantities
|
Hearing aids,
cameras, wrist watches
|
Why
are mercury button cells still on the market?
Certain cameras and
electronic devices have specific voltage requirements and rely on the
use of mercury button cells to work properly as they need a more stable
voltage during discharge. Nevertheless, because of the battery's mercury
content (30% pure mercury) the composition of these cells is considered
a danger to the environment if not disposed of in a correct manner. For
this reason all battery manufacturers will have ceased production of mercury
cells by the turn of the century. With the development of the zinc-air
button cell, manufacturers like Varta have achieved their goal for one
essential application: hearing aids.
Why
does a zinc-air battery have a self-adhesive film?
The zinc-air battery
is activated if exposed to air. The activation occurs as soon as the self-adhesive
film is removed, thus opening the battery's air access hole(s) to the
atmosphere. The oxygen in the air is electrochemically activated via an
air electrode inside the battery. As the air electrode requires far less
volume than, e.g. a silver-oxide electrode, far more zinc can be accommodated
inside the button cell housing. Thus the zinc-air cell has a very high
capacity, far more than all other button cells and even lithium cells.
In addition the zinc-air battery offers a high load capability.
What
temperature range can button cells be used in?
Button cells should
preferably be used in a temperature range from +10°C to +35°C. Permissible
minimum and maximum temperatures are -10°C and +65°C respectively. The
temperature range for zinc-air button cells is more restricted.
What
are coin cells?
Coin cells (coin-shaped
cells) are also button cells. However, their diameter/height ratio is
particularly large. All coin cells use lithium
systems. Their smallest
diameter is 10 mm, their largest 30 mm. Heights range from 1.2 mm up to
5.4 mm.
Are
there any rechargeable button cells on the market?
Yes, there are various
types of rechargeable button cells available, primarily using nickel-cadmium
and nickel-metal-hydride technologies. Rechargeable lithium button cells
have recently been introduced to the market. At present they are generally
sold with their devices they are used in. It is currently difficult to
purchase them through normal retail channels.
Automotive
Batteries (SLI-Batteries)
What
does "SLI battery" mean and how is the battery constructed?
An SLI battery is
an automotive battery and stands for Start, Light
and Ignition. Two substances dominate a standard SLI
battery: lead and sulfuric acid. The positive electrode consists of lead-dioxide,
the negative electrode is composed of finely distributed sponge lead.
The sulfuric acid forms the electrolyte, ensuring the flow of ionic current
between the battery's electrodes. The sulfuric acid's maximum conductivity
is obtained at a gravimetric density of 1.28 kg/l. This is a typical acid
density.
In an SLI battery,
positive and negative electrodes are alternately welded to electrode stacks
and set into the battery housing. A separator is placed between them to
electrically isolate the positive and negative electrodes from each other.
Six of these series-connected electrode stacks form a 12V battery.
What
happens when an SLI battery is charged?
As soon as the engine
starts running, the battery charging process is initiated by means of
the alternator. The result of the recharging process is that the lead
sulfate formed during the discharge process will again become lead dioxide,
lead and sulfuric acid, thus restoring the necessary chemical energy to
be converted into electrical energy in future use.
The optimal charging
voltage of the car's voltage regulator is 14.2V. If the regulator voltage
is set too high, water will be electrolyzed. This lowers the electrolyte
level over time. If the regulated voltage is set too low, the battery
will not be charged properly, this also shortening its life span.
Audio/Video/Photo
Equipment
When
are alkaline-manganese primary batteries preferable to accumulators to
power a portable cassette player?
If a portable cassette
player or portable Compact Disc player is used only occasionally, it is
preferable to use alkaline-manganese primary batteries instead of accumulators
because of the letters' relatively high self-discharge. However, if this
type of equipment is to be used more frequently, e.g. on a daily basis,
it may be advantageous to use rechargeable nickel-metal-hydride batteries.
However, it is important to remember that they need to be recharged regularly,
since they cannot match the single-charge capacity levels of alkaline-manganese
primary batteries. When using accumulators intensively it is advisable
to ensure you always have a fully charged spare set at hand.
How
long do video/camcorder batteries last?
This depends very
much on the type of camcorder and the kind of usage. The operating time
of nickel-metal-hydride accumulators is up to 40% longer than that offered
by traditional nickel-cadmium accumulators of the same weight and volume.
This is due to the higher capacity. Typical operating times for the latest
nickel-metal-hydride range from 1 hour to 6.5 hours dependent on the wattage,
accessories and age of the camera and the capacity of the battery.
What
is a universal video/camcorder battery?
This is a rechargeable
battery designed for both 8 mm and VHS video camcorders. One side of this
battery carries the contacts for an 8 mm camcorder and the other side
those for the VHS system. These universal accumulators or "multifits"
were developed to fit about 1,000 different camcorder models; there are
some 8 mm or VHS camcorders, however, which cannot use these "multifits".
In this case, an expert should assist in selecting an appropriate power
pack.
May
a video accumulator remain in the camcorder after use ?
If the video camcorder
is not in use for an extended period of time it is better to remove the
power pack from the camcorder and to store it in a dry and cool place.
If this is not done, a minimum amount of current will continue to be taken
out of the battery by the camcorder system - even if the camcorder is
switched off - which may shorten the battery's service life.
What
about an accumulator that has not been used for a long period of time?
An accumulator standing
idle for a long period of time may be totally discharged due to its inherent
self-discharge. The accumulator must be cycled a number of times (charged
and discharged) to restore its original capacity, i.e. operating time.
Why
are lithium batteries particularly suited for cameras?
Lithium batteries
offer relatively high volume-specific energy (approx. 800 mWh/cm³). In
addition, lithium batteries which have spirally wound large-surface electrodes
have a high load capability and high capacity retention during storage.
Both the lithium battery's longer operating time and its higher cell voltage
of 3V are important in camera applications. The latest generation of cameras
is fitted out with numerous automatic functions, which means increased
energy coupled with relatively high load requirements. Lithium batteries
of this type are a particularly good choice for today's cameras.
Accumulators are rarely
used in cameras, why?
A sudden voltage break-down
at the end of discharge is typical for accumulators. When an accumulator
is used to operate a camera, especially those of former design, this can
lead to an annoying situation if the flashlight suddenly stops working,
and an important scene to be photographed remains undocumented. A situation
of this kind can of course easily be avoided by checking the battery's
state of charge well in advance. Another way of avoiding such an unexpected
situation is to use alkaline-manganese batteries (ALKALINE). Typical for
this primary system is that - during discharge - its voltage and thus
load capability decrease gradually, so that the user recognizes the end
of discharge in good time and has the opportunity to change the battery.
Some of the newer generation of cameras have eliminated the above problem
with accumulators.
Which
battery type should be used for remote control devices?
A remote control device
should only be operated by the battery stipulated in its battery compartment.
Different zinc-carbon batteries are available for different remote control
devices. They can be identified by their IEC designation. Commonly used
batteries include the R03 (AAA, "Micro"), R6 (AA, "Mignon") and the 9V
Bloc 6F22. A better choice is the alkaline versions of these batteries
which offer twice the operating time of the zinc-carbon battery. They
can be identified by their IEC designations LR03, LR6 and 6LR61. Nevertheless,
because of the relatively low current required by this application, zinc-carbon
batteries still remain a good and economical alternative.
Interchangeable accumulators
may - in principle - be used as well. They are, however, less recommendable
for this application because of their relatively high self-discharge,
which requires repeated charging, thus rendering this type of battery
rather impractical.
Watches
/ Hearing Aids
What
types of batteries are used for watches?
There is a wide range
of button cells available for watches. The preferred electrochemical system
is silver oxide. Varta offers 40 different types of this system. The type
of battery to be used is listed in the watch's operating instructions.
In general, it is important to remember that analog watches (watches with
hands) and simple digital watches are powered by "low drain" batteries.
They are extremely leakage-proof. However, due to their higher internal
resistance, they do not comply with the current requirements of multi-functional
watches, which need "high drain" batteries to operate their multiple functions
(i.e. alarm, illumination of the watch's face etc.).
What
other battery systems can be used for watches?
In addition to the
silver-oxide system, alkaline-manganese and lithium-manganese systems
are also used in watches. The alkaline-manganese button cell is most commonly
used for low price watches. This battery can later be replaced by an identical
sized silver-oxide button cell. The advantage of the silver-oxide button
cell is its constant operating voltage (highly accurate time-keeping)
and higher capacity (longer operation).
Another category of
watches uses lithium coin cells, which may also be equipped with multiple
functions. A typical coin cell for this purpose is the CR2025, with a
diameter of 20 mm and a height of 2.5 mm. In total there are more than
12 different sizes (different diameter and height).
Is there an environmentally
benign alternative to mercury batteries which satisfies the high power
requirements of hearing aids?
Yes, Varta has launched
a new zinc-air battery on the market, named "ZincAir Top", which can unquestionably
replace mercury batteries in all hearing aids. The load capability of
this battery is higher than that of the traditional zinc-air battery and
even higher than that of the mercury battery. "ZincAir Top" batteries
from Varta can only be purchased from authorized hearing aid dealers.
How
long do button cells for hearing aids last before they run down?
This depends on the
type and usage of the hearing aid. On a 12 hours/day "on" and a 12 hours/day
"off" regime, the "ZincAir Top 675" button cell (IEC: PR44) provides enough
energy to operate a hearing aid for two weeks, whereas the small battery
of type 10 or 230 (IEC : PR70) can be used for only a few days following
activation.
Are
rechargeable button cells available for use in hearing aids?
Small-sized rechargeable
button cells for hearing aids would not be practical for the consumer.
The operating time would be quite short and the button cell would need
recharging several times a day. An exception is battery type 675, which
may be replaced by an interchangeable accumulator. The accumulator must,
however, be recharged after eight hours' use.
Telecommunications
How
long does a battery last for a mobile telephone?
This depends on the
type of power pack, and the age and type of mobile telephone. This is
measured in standby time and talk time, where actual conversation needs
more energy than keeping the telephone in standby mode.
When
is it preferable to use a high-capacity accumulator for a mobile telephone
and when is it better to use a medium-capacity "slimline" battery?
High-capacity accumulators
deliver a longer operating time than slimline accumulators; however, they
are heavier and larger. Slimline accumulators are lighter and are especially
designed to fit mobile telephones, but offer a shorter operating time.
This aspect should be kept in mind when selecting an accumulator for a
mobile telephone.
What
is the service life of accumulators for cordless telephones?
The service life is
2 to 3 years, sometimes longer. The accumulator needs to be replaced if:
- the talk time falls
from charge to charge;
- the reception becomes
indistinct;
- the allowable range
between cordless phone and base unit is reduced.
Is
it true that a cordless telephone should not be put back onto the base
unit after each single use?
With cordless telephones
equipped with conventional nickel-cadmium accumulators, putting the telephone
back onto the base unit after each single use builds up the battery's
memory effect and thereby cuts the operating time. Nickel-metal-hydride
batteries from Varta have virtually no memory effect. The same is true
for button cells. Nevertheless an occasional complete discharge is to
be recommended in order to restore the battery's original capacity and
discharge behavior.
Automobiles
What
role does the battery play in providing electrical power to an automobile?
An increasing number
of electrical consumers used in modern automobiles depend on just one
SLI battery. This is particularly problematic if the engine is not running,
i.e. if the battery is not being charged. Such installations include heated
seats, heated rear window, electric window openers, air conditioner, radio,
mobile telephone, reading lights etc. The demand for inside comfort is
rising constantly. As a result, the performance requirements for an efficient
SLI battery are also increasing. However, a battery whose primary task
is to ensure a reliable engine start cannot be expected to carry unlimited
additional loads.
Concepts are therefore
being developed which, in the future, will provide vehicles with several
batteries and cable networks.
How
must SLI-batteries be installed and removed?
- Prior to installation
or removal: switch off all electrical consumers.
- Essential for installation:
the battery must be installed in such a way that it is mechanically
secured. Its degassing vents must not be covered. In the case of centrally
vented batteries, the ventilation hose must be connected.
- Essential for removal:
when detaching the electrical connections, first remove the ground cable
from the negative terminal. Then disconnect the cable from the positive
terminal. This avoids short-circuits.
How
can I start the car when the battery is flat?
Two things are necessary
- a second car and two jump leads for the positive and negative terminals.
An electrical connection via jump leads from an SLI battery being charged
by the running motor to the SLI battery of the car that won't start is
normally the simplest solution. The start procedure is to be carried out
in six steps.
First step: switch
off all electrical devices in the car to be started.
Second step: connect
one end of one lead to the negative terminal of the charging battery and
the other end to the chassis of the vehicle which won't start.
Third step: use the
other lead to connect the positive terminals of the two batteries.
Fourth step: wait
a few minutes to allow the flat battery to be charged a little.
Fifth step: start
the engine of the troubled car.
Sixth step: disconnect
the two jump leads in precisely the reverse order. The car bodies must
not touch each other when performing this procedure.
Note: please refer
to the operating manual for your vehicle!
What
kind of battery problems can occur during use?
If an SLI battery
is not properly maintained, battery failure may be the result. Unclean
terminals may cause leakage currents, leading to energy loss. If a car
is predominantly used in "stop and go" traffic while using installations
like air conditioning systems, heated seats, heated front and rear window
etc., the battery may be discharged excessively, thus causing difficulties,
e.g. when trying to start the car in winter!
What
kind of maintenance does an SLI battery require?
All automotive batteries
require a certain amount of maintenance.
1. The surface
of the battery should be kept clean and dry, otherwise leakage
currents can build up, causing additional loss of charge. Batteries
and terminals should be periodically checked to ensure a tight fit,
and should be tightened if necessary. For automotive batteries with vent
plugs, the following should be noted:
2. Battery
fluid levels should be checked regularly. During the warmer months
of the year, water consumption is normal. If consumption increases noticeably,
the control voltage should be checked by a specialist. If the battery
fluid level is too low, it should be topped up with purified water.
Acid should never be used. When storing automotive batteries, the following
should be noted:
3. Batteries should
always be kept as fully charged as possible to prevent the formation of
large lead sulphate crystals. Batteries should never be stored
in discharged (or partially discharged) state!
4. Charged batteries
in storage should be checked regularly, and should be charged when the
acid density falls below 1.20 kg/l.
Deep
Cycle Batteries For Mobile Applications
What
is the vehicle electrical system?
Vehicle electrical
systems can be found on airplanes, ships, house trailers and cars. It
encompasses all kinds of electrical installations, consumers, wiring and
cables, as well as the battery, generator and starter.
Why
is it not advisable to use an SLI battery as a power source for long periods?
SLI batteries are
used repeatedly for applications they never were made for, e.g. as power
supplies for house trailers, to provide extra electrical energy for mobile
ambulances (lights etc.), as energy supplies for boats, or as backup batteries
for computers.
An SLI battery's primary
task is to provide a high power output for a short period of time necessary
to start a combustion engine. In order to provide these high current outputs,
large electrode surface areas are necessary. This is realized by using
many thin electrodes connected in parallel.
Permanent cycling,
i.e. charging and discharging, of 60-80% of the rated capacity at medium
currents over a longer period of time can produce strong mechanical forces
within the thin plates of the SLI battery. These forces may cause the
active mass to separate from the electrode grid and thus lead to premature
wearing of the battery.
Therefore, when discharging
60-80% of a battery's rated capacity, special batteries should be used
which are designed for this type of application.
For the "semi-traction"
application area, Varta offers two distinct technologies, i.e. sealed
batteries using a gelled electrolyte and wet batteries employing specially
designed electrode stacks with liquid electrolyte.
In order to guarantee
optimal battery life, "gel" batteries may be discharged (cycled) up to
60%, whereas wet batteries may be discharged up to 80%. Thus - depending
on technology - 60% or 80% of the rated capacity will be available.
Deep discharges should
also be avoided with these batteries. Deep discharges which occur when
capacity is withdrawn beyond the minimum voltage limit cause the battery's
service life to be shortened. In order to protect the battery from deep
discharge a "deep discharge protector" should be used.
Leak-proof
semi-traction batteries, are they available ?
Yes. They use a gelled
electrolyte and are leak-proof even when stored upside down. These batteries
are used in boats, in house trailers or in small electric vehicles (golf
buggies, wheelchairs etc)
How
can I select the correct capacity for a semi-traction application ?
The choice of the
correct battery capacity is best made with the help of a checklist. Make
a list of all the electrical consumers on your house trailer or boat.
The power consumption of the individual installed units can be found in
the relevant manufacturer's data sheet and should be given in watts. Dividing
the power consumption by the voltage (12V or 24V) gives you the current
in amperes. Now estimate the usage of the individual consumers in hours,
total these and calculate the capacity required in ampere hours.
In order to determine
the battery capacity actually needed, the estimated result should be multiplied
by a safety factor. A safety factor of 1.5 is recommended for "wet" batteries,
i.e. batteries using liquid electrolyte. For gel batteries, as in our
example, a safety factor of 1.7 should be used. In this way the risk of
a deep discharge can be avoided provided the battery is properly maintained.
When applying the
safety factor, the resulting capacity may lie in-between two existing
Varta battery types. In this case the battery offering the higher capacity
ought to be chosen.
Example:
Capacity requirement/day
51.5 Ah x safety factor 1.7 = 87.55 Ah (K20)
Capacity (K20) 89.25
Ah x conversion factor 0.85 = 74.4 Ah (K5)
Note: When comparing
batteries, keep in mind that K5 or K20 means a capacity with a 5 or 20
hours discharge rate.
Use
of Solar Energy
What
are the advantages of a solar battery?
During recent years,
use of environmentally benign solar energy has increased. Solar energy
systems are easy to install, easy to expand, and easy to disassemble.
They are economical as well, since there are no energy costs during operation.
In addition, solar energy systems are subject to virtually no mechanical
wear. A solar energy system requires a reliable solar battery for charge
acceptance and storage. The solar battery from Varta is characterized
by:
- high charge acceptance
- durability in cycle
operation
- good rechargeability
- virtually no maintenance
What
is the solar battery´s rate of self-discharge?
Compared with other
rechargeable systems, solar batteries with a liquid electrolyte have a
remarkably low self-discharge of only 10% per month (approx. value, determined
at 25°C).
Which
components are needed to operate a solar energy system?
The two basic components
of a solar energy system are the storage battery and the solar cells.
The solar battery from Varta is a grid plate battery with liquid electrolyte.
Its performance characteristics (e.g. cycle life and deep discharge capability)
are tailored to the requirements of a small solar-electrical system. Solar
cells are available in thin rectangular-shaped elements (10 x 10 mm).
Depending on the voltage needed to charge the solar battery system, a
relevant number of solar cells are connected in series (module).
What
percentage of primary energy can be stored and delivered by a solar battery,
and what kind of application is this system suitable for?
The efficiency of
solar energy conversion into electrical energy is currently 11%-14%. The
charging efficiency is approx. 90%, meaning that when X ampere hours are
removed from the battery a charge of 1.1 X ampere hours must be recharged
into the battery in order to maintain the original level of charge.
Small solar power
systems can be used in cottages, vacation homes, trailers and boats, as
well as in isolated areas to operate e.g. an emergency telephone. Solar
systems offer a useful, environmentally benign, low-maintenance and silent
source of energy.
What
maintenance do solar batteries require?
During normal use,
the battery should be checked and maintained only once a year. To ensure
that the charge conditions meet the requirements, the electrolyte should
be checked with a hydrometer (acid density of the charged battery: 1.28
kg/l at 25°C). Also to be checked is the battery’s open circuit voltage,
which should be 2.12 V/cell (if fully charged).
Batteries
and Environmental Protection
Do
batteries harm the environment?
Today nearly all consumer
batteries, especially primary batteries, are free of mercury and cadmium.
On the other hand, heavy metals are still essential components in mercury
batteries, rechargeable nickel-cadmium batteries and lead-acid accumulators.
These metals may cause damage to the environment if disposed of improperly
and in large quantities.
The battery industry
is working to develop alternatives to replace mercury, cadmium and lead
wherever possible. Alternatives are already available for a large number
of applications (e.g. nickel-metal-hydride and zinc-air systems). Other
new technologies are already in an advanced stage of development.
Mercury oxide, nickel-cadmium
and lead batteries are already being collected and recycled in Europe.
Some of the raw materials are reused in the manufacture of new batteries.
Varta has been recycling used lead batteries in its own recycling plant
for many years. For example almost 100 % of lead batteries in Germany
are collected and recycled.
What
do I need to know about returning rechargeable batteries?
Rechargeable batteries
of any type should only be placed in dealers' collection boxes or returned
to the local authorities when they are discharged. When the equipment
stops working and says "battery dead", or if it fails to work properly
after a long period of use, then the batteries are discharged. If you
are not sure whether the battery is completely discharged, you should
cover the poles of the battery with a piece of sticky tape or return the
battery in a plastic bag. It is the responsibility of the dealer and manufacturer
to dispose/recycle the various electrochemical systems as per the country
of sale regulations.
The
future of Batteries
Are
batteries becoming smaller like the equipment they are made for ?
Yes - although there
are limits - the trend towards smaller batteries is continuing. Among
standard round cells the smaller sizes, i.e. the Penlight (R6, AA) and
small Penlight (R03, AAA) are becoming more and more popular. These two
battery types currently account for 70% of sales, while the demand for
larger standard round cells like Baby (R14, C) and Mono (R20, D) is decreasing.
New rechargeable battery systems like nickel-metal-hydride and lithium-ion
enable the use of smaller batteries which offer the same amount of energy.
In addition, more and more devices are being operated with button cells.
Rechargeable button cells can be expected to gain more and more in importance
in future.
Which
batteries will dominate the market in years to come?
By the year 2000,
rechargeable portable batteries will probably have a larger market share
than primary batteries. The dramatic growth predicted for the years to
come will be primarily in the area of rechargeable power packs. Camcorders,
mobile and
cordless telephones, notebooks and multimedia devices with picture,
sound, and speech transmission will have found their place in the majority
of households
What
are "intelligent" accumulators (smart batteries)?
Intelligent accumulators
are equipped with an electronic chip which is not only responsible for
the device's energy supply, but also controls its main functions. This
type of accumulator indicates the charge remaining, the number of times
the accumulator has been charged already, its temperature etc. Intelligent
accumulators are not yet available on the market, but will most definitely
play a major role in the not too distant future - particularly in the
field of power packs for camcorders,
cordless
and mobile telephones and notebooks.
Which
new portable energy sources will be available in the future? What consequences
will they have on the battery market?
There is no alternative
to a battery for storing and providing electrical energy. It will remain
our most important mobile energy source, together with solar energy (via
solar cells). No other energy system offers the convenience of the battery.
The battery for a wrist-watch, for instance, can in principle be replaced
by kinetic energy created by body movement; however, if the wrist-watch
is not used for a certain period of time, it will cease to work, i.e.
the hands won't move.
Will
battery systems continue to exist in today’s large variety, or will
there be a system in future that will accommodate all our needs?
Each one of today's
battery systems is a specialist in its own right, able to fulfill a specific
task better than any other battery system. They all are specialists either
in terms of value for money, high capacity, high energy density, long
shelf life, high or low operating temperatures, environmental compatibility
or economical, environmentally benign recyclability. A battery system
capable of combining all such characteristics is unlikely ever to be available.
Recommendations,
including those from the IEC:
- Keep batteries
out of the reach of children, especially those that may be ingested
(IEC).
- In the case of
ingestion of a cell or battery the person involved should seek medical
assistance promptly (IEC).
- Equipment intended
for use by children should have battery compartments which are tamper-proof
(IEC).
- The circuits of
equipment designed to use alternative power supplies should be such
as to eliminate the possibility of a primary battery being charged (IEC).
- It is of extreme
importance that batteries be inserted into equipment correctly with
regard to polarity (IEC).
- Do not attempt
to revive used primary batteries by heating or other means. Primary
batteries must not be charged as this can cause leakage, explosion or
fire (IEC).
- Recharge and maintain
accumulators according to the manufacturer’s instructions; employ
only approved, high quality chargers designed for the intended battery
system.
- Do not immerse
batteries into water* and do not store them in a damp place; instead
store them where it is dry and cool (*unless the packaging is absolutely
water-tight) (IEC).
- Do not dispose
of batteries in fire; do not try to open batteries or to solder or weld
batteries yourself (IEC).
- Only use the type
of battery recommended by the equipment manufacturer. Follow the instructions
and symbols on the battery compartment.
- Do not short-circuit
batteries (IEC).
- Replace all batteries
of a set at the same time (IEC).
- Newly purchased
batteries should not be mixed with used ones, batteries of different
electrochemical systems, grades or brands should not be mixed either
(IEC). This also applies to rechargeable batteries.